Programme Tracks and Multi-disciplinary themes
It seems that ‘one drug for all’ may become rarer, mainly because future interventions will be context appropriate in terms of disease subclassification, patient genetics and treatment history, drug pharmacokinetics, pharmacodynamics, etc. Disease variants and their stages of progression are being defined at ever higher resolution.
An increasing number of therapies are targeted to specific patient profiles in order to achieve increased effectiveness and improved tolerance. Point-of-care companion diagnostic kits and sophisticated drug vectorization technologies are becoming more common.
Engineers and life scientists are working together to develop new sensors and intelligent systems to monitor and analyze ever more closely changes in biomarker status in disease and under treatment, notably using “omics” approaches.
At the level of communities, we see the promise of continuously improving, learning health care systems, although the rising costs of new medicines represent a challenge. Optimal access to innovative medicines will require the integration of baseline health data, treatment outcomes and drug efficiency analysis to support decision making.
These considerations will have major effects on how we plan all aspects of drug discovery, development, fabrication and usage.
This will be a completely virtual event; the speakers’ presentations will be made available on video before each session, so that the session itself can be a vibrant live panel discussion with the speakers.
Together with a virtual poster gallery, the videos and the live panel discussions, this concept will offer an excellent opportunity for our worldwide audience to be part of a truly international pharmaceutical sciences event!
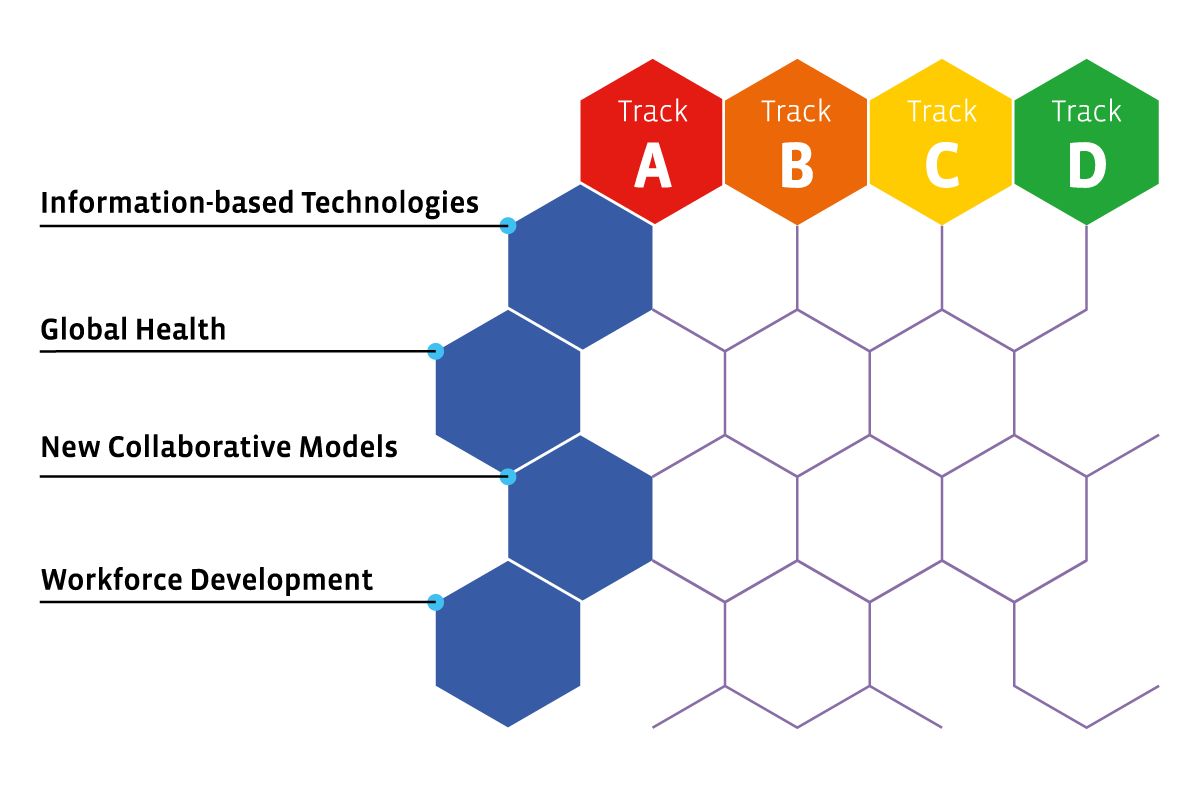
The scientific programme covers recent developments in the pharmaceutical sciences from target to market. The scientific programme is structured as a matrix in which the different columns are the tracks in the scientific programme and the rows reflect the multi-disciplinary themes that may be relevant to more than one track. The figure shows the overall structure of the matrix and highlights how the successive tracks correspond to key phases in the continuum of drug R&D and use. The different tracks in the conference as reflected in the columns are:
Track A = Novel concepts – From diseases to clinical candidates
Track B = Advanced strategies – Meeting the challenges of optimal manufacture and delivery
Track C = Effective translation – From predictive experimental models to optimized clinical studies
Track D = Societal impact – Developing context-appropriate solutions
Track E = Multi-disciplinary themes
Conference Matrix
Programme Tracks
Track A Novel concepts
From diseases to clinical candidates. Participants will explore new concepts in our understanding of disease mechanisms, both at the molecular level as well as systems biology levels, and discuss cutting edge approaches to best discover and design appropriate therapeutic modalities, from small molecules to biologics, cell-based therapies or gene-targeted approaches.
Track B Advanced strategies
Meeting the challenges of optimal manufacture and delivery. Participants will discuss how to meet the demands of individualized medicine and global health with cutting-edge formulation, bioanalytical technologies and manufacturing strategies.
Track C Effective translation
From predictive experimental models to optimized clinical studies. Participants will discuss how scientific, technological and regulatory developments can increase success rate in clinical trials and help better individualize treatments, with new predictive experimental models and innovative methods for monitoring treatment exposure and response.
Track D Societal impact
Developing context-appropriate solutions. Participants will discuss the contribution of real-world evidence to the evaluation of therapeutics, in order to better address individual patient needs as well as public health issues with the efficient implementation of therapeutic innovations in a context-appropriate manner in communities around the world.
Multi-disciplinary themes
Information-based technologies
Our ability to collect and analyze data is growing exponentially, creating new opportunities to monitor, understand and influence biological processes. This theme refers to the growing use (or potential impact) of big data in pharmaceutical sciences and drug development. It includes the increasing reliance on smart devices that can collect data (remotely or not), the aggregation of large databases and the development of tools to analyze the data in order to generate smart data out of big data. Of these tools, artificial intelligence (AI) is a prominent technology that is gaining momentum and is poised to revolutionize pharmaceutical sciences. What are the emerging applications of AI and other big data tools in pharmaceutical sciences?
Global Health
This theme addresses the need to find new ways to deliver innovative treatments to patients around the world in a manner that is sustainable and appropriate for local conditions, in order to address the challenges of neglected diseases in low- to mid-income countries, the response to emerging pathogen threats.
New collaborative models
This theme aims to promote a healthy regulatory and business ecosystem stimulating innovation in drug R&D to address individual and global needs, from open access or patent-based research, public – private partnerships and international consortia to support research in neglected, rare or intractable diseases.
Workforce development
This theme aims to identify the optimal conditions for professional development among pharmaceutical scientists and new types of experts into clinical practice, such as data scientists and digital health technology experts, for effective knowledge transfer from science to pharmacy practice, and for training the next generation of research and healthcare professionals.
